COVID-19 MONOCLONAL ANTIBODY IV Therapy
Convenient At-Home COVID-19 Monoclonal Antibody IV Therapy
At-Home Monoclonal Antibody Therapy is now available from Reset IV for patients who have tested positive for COVID-19, been exposed to COVID-19, or are experiencing mild to moderate symptoms!
FDA-EUA APPROVED
Monoclonal Antibody IV Therapy can help prevent and reduce symptom severity, and may help shorten the duration of COVID-19.
Eligibility
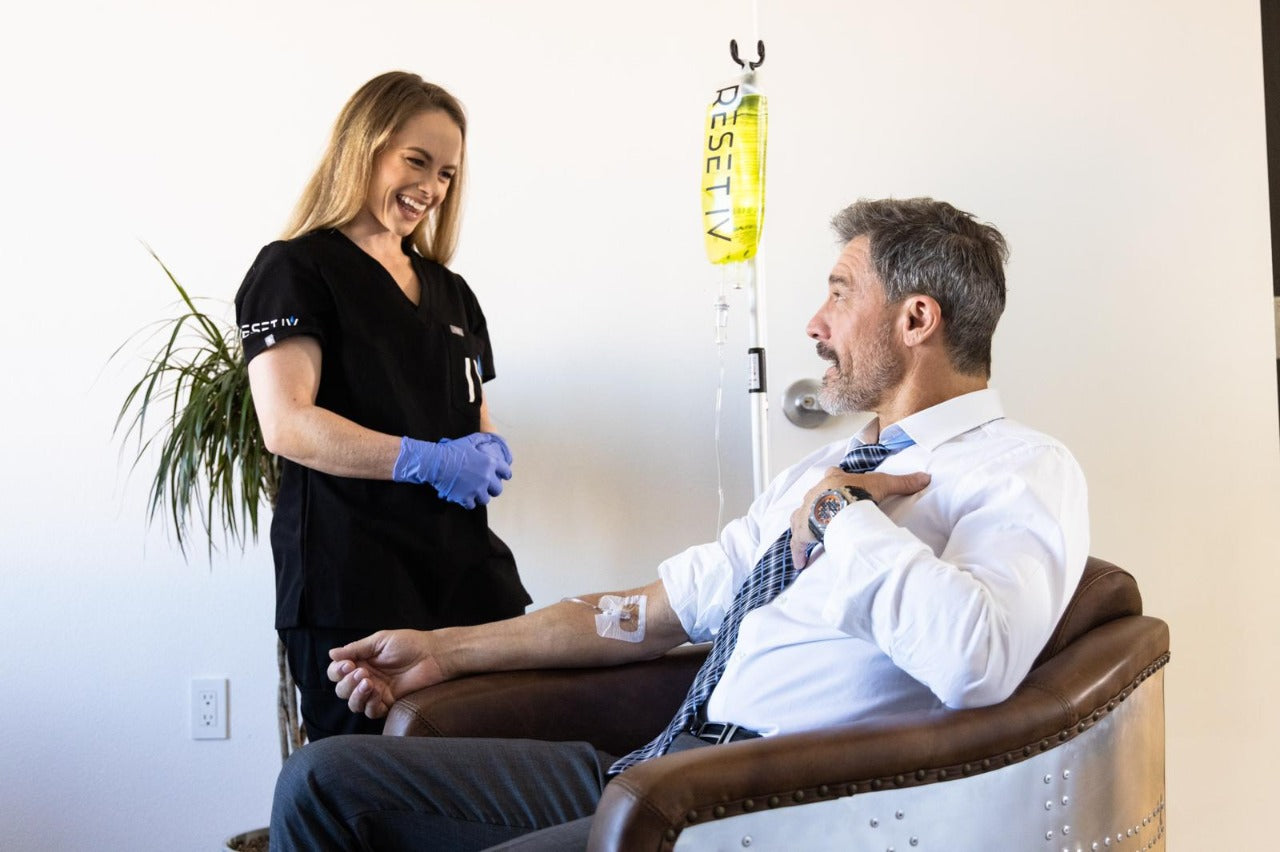
Monoclonal Antibody Therapy in the comfort of your home
Are You Eligible?
If you’re 16 years of age or older,
AND you have tested positive for COVID-19 within the past 7 days,
OR you have been exposed to COVID-19 within the past 7 days,
OR you have had an onset of symptoms within the past 7 days
then you qualify.

3 Simple Steps
Your Covid-19 Safety Plan in 3 Simple Steps
1. See If You Qualify
Fill out the form below, or Call us at (844) 934-1922 RESETIV to see if you’re eligible for Monoclonal Antibody Therapy.
2. Review & Schedule
If you qualify, you can easily book an at-home appointment below.
3. Monoclonal Antibody Treatment
A medical professional will come to you to administer the IV treatment. The IV drip typically takes about 30-40 minutes. Our nurse will then monitor you for 1 hour after treatment.
Complete the form below, or call to see if you qualify today (844) 934-1922 RESETIV .
BOOK APPOINTMENT
Book Monoclonal Antibody IV Therapy
Before booking, please complete questions below to check if you qualify. If you have any questions, please call (844) 934-1922 RESETIV
FREQUENTLY ASKED QUESTIONS
FREQUENTLY ASKED QUESTIONS
Covid-19 monoclonal antibodies are specialized proteins that mimic human antibodies. They’re almost identical to the already existing immune response proteins you already have inside of you. Adding extra proteins through our coronavirus IV treatment helps your body fight off harmful viruses like Covid, which is a SARS-CoV-2 type virus. They also help reduce the symptoms and spread, if you’re infected.
despite their vaccination status.
https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-
antibody-products/anti-sars-cov-2-monoclonal-antibodies/
The FDA has approved mAbs therapy for emergency use. Like any injection or infusion, there are potential side effects such as pain, bleeding, bruising, soreness, swelling, fever, chills, exhaustion, nausea, headache, and possible infection at the infusion site. In rare cases, people may develop hypersensitivity and allergic reactions during or after an antibody infusion. This is why we have trained medical professionals monitor you for 1 hour after your treatment.

